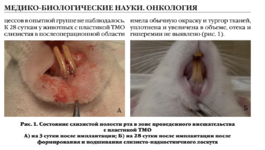
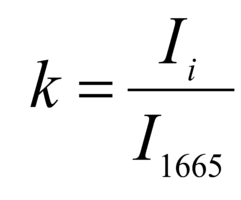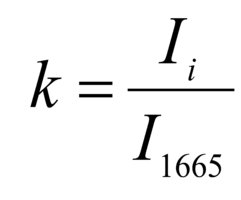+7 (929) 727 53 60 Травматология / Ортопедия
Optical estimation of the composition of bone implants during processing
P.E. Timchenko1, E.V. Timchenko*1, L.T. Volova2, O.O. Frolov1, V.D. Meseyarakov1, Pugachov E.I.2
1 Samara National Research University, 34, Moskovskoye shosse, Samara, 443086, Russia
2 Samara State Medical University, 89 Chapayevskaya St., Samara, 443099, Russia
This paper presents the results of the experimental study of the use of ultrasonic treatment for the production of bone
implants using Raman spectroscopy (RS). It shows that Raman spectroscopy can be used to estimate a change of bone
implants composition during their processing. It was shown that ultrasonic treatment reduces the concentration of cellular
components in the bone tissue. The conclusion was based on the two-dimensional analysis of the given ratios.
Keywords: Raman spectroscopy, spectral analysis, optical coefficient, bone implant, demineralization, ultrasonic
treatment.
The issue of bone tissue restoration after fractures is an important medical and social problem of the modern medicine [1]. According to the World Health Organization, fractures of the proximal femur and vertebrae are in fourth place among all
causes of disability and mortality after cardio-vascular diseases, cancer and diabetes [2]. Fractures of the proximal femur are detected annually in 100.9 cases per 100 thousand of the population and due to the ageing of people it is expected that the number of fractures will increase from 1.7 million in 1990 to 6.3 million in 2050 [3]. The number of patients in need of operations to restore the completeness of the bone is quite large. For Russia or the United States, this figure is more than 1 million people annually (of which 200-300 thousand – hip and knee prosthetics) [4, 5].
In recent years, implantology has been actively working to create biomaterials for forming microenvironment, osteogenesis and creation of a mineralized surface that plays the role of a natural connection between the new tissue and biomaterial, which allows for the successful treatment of pathologies of the musculoskeletal system [6]. Bone implants are used in reconstructive surgery to restore structural of bones and increase osteogenic potential of bone tissue [7]. Demineralized lyophylized bone biomaterial compared to bone alloplastic implants, has a more pronounced osteoinductive, however, its performance and structural strength also depend on the method of preparation of the implant.
Bone implants should be nonimmunogenic, bioresorbable and have certain physical,mechanical and chemical-biological properties [8, 9]. Therefore, the use of bone-forming donor implants is limited by the risk of infection and immunological rejection of the graft [10].
In the process of creating implants there is contamination of their surface, which depends on the technology of their manufacture and the method of treatment of the intraosseous surface. Cleaning of the implant surface can be carried out by chemical methods, which leads to the removal of donor cells and the destruction of nucleic acids. However, it may reduce the content of main components of biomatrix: denaturation of proteins and deformation of the collagen matrix, which leads to a violation of the biological properties of bone tissue [11].
An alternative method of primary sterilization of the material and viral inactivation in the process of obtaining implants is ultrasonic treatment. Removal of bone marrow elements and fat from spongiose allows to prevent antigenic and immunological contamination with saving of all necessary components for osseointegration [12].
The quality of the implant directly depends on the content of hydroxyapatite, collagen, non-collagen proteins and sulfated glycosaminglican, which are the basic osteoconductive components of the intercellular matrix and their destruction during the treatment of bone tissue can lead to loss of conductive and inductive properties [13]. Therefore, control of the composition of the allogenic bone tissue in the process of receiving and processing is a very important issue in case creating an optimal protocol for the manufacture of the implant.
Optical methods of biomaterial control at the modern level of science and technology development, in contrast to histological and biochemical analysis, have enough opportunities to successfully solve this problem, due to its simplicity, non-invasive and efficiency. Raman spectroscopy (RS) has a number of advantages compared to other analytical methods [14,15,16] and allows to carry out non-destructive, non-invasive quantitative and qualitative analysis of the composition of biological objects and provides information about molecular structure with high spatial resolution.
For example, the authors of [14,16-19] used the Raman spectroscopy method to estimate the content of main components of human bone tissue – hydroxyapatite and type I-III amides using the mineral/organic matrix ratio. Comparing the amplitudes of the Raman peaks at the wave numbers in the region from 400 to 1800 cm-1, the authors revealed significant changes in the signals for samples with different degrees of demineralization. The analysis found that the presence in the spectrum of certain peaks and their amplitude can be judged on the composition, degree of mineralization and structural strength of bone samples. The main changes in the spectrum are observed at the wave numbers 428 cm-1, 585 cm-1, 960 cm-1, 1065 cm-1 corresponding to phosphate and carbonate ions in the hydroxyapatite molecule. It has been established that Raman spectroscopy can be used for non-invasive and non-destructive analysis of bone implants during their treatment.
The aim of the work: to study the effect of ultrasonic treatment on the composition of bone implants using Raman spectroscopy.
MATERIALS AND METHODS RESEARCH
Objects of the research were 48 solid spongy bone bio-implants in the form of a cube 5*5*5 mm, made using the "Lioplast"® technology ((ТУ-9398-001-01963143-2004). For comparison, the samples were grouped according to the method of preparation: allogeneic (cadaveric) and intraoperatively resected. In each group the samples were divided into 4 subgroups according to the degree of demineralization and ultrasonic treatment (8 groups of samples).
The objects of the study were samples of hard spongy bone tissue (size 5*5*5 mm). The studied samples were made using the technology "Lioplast"® (TU-9398-001-01963143-2004). Spectra were recorded for each sample on each side of the cube at three different points. In the process of production of bone bioimplants at the first stage, low-frequency ultrasonic treatment (24-40 kHz for 2-3 minutes) was carried out, as a result of which the samples of biomaterial were degreased and the bone marrow elements were removed from the inter girder spaces of the bone, while due to the technical and physical features of the treatment, the destruction of the bone tissue itself did not occur. Then, to obtain demineralized biomaterial, bone tissue was placed in a solution of hydrochloric acid 1,2 normality for a time, depending on the localization of bone biomaterial clipping and age criteria of the donor. Further, the obtained demineralized biomaterial passed the final stage of its production – the process of lyophilization by means of the sublimation unit ALPHA 2-4 LSC, which consisted in the sublimation drying of samples. In this process, there was no loss of structural integrity of proteins and microelements of bone tissue. Control samples were not subjected to the process of demineralization and ultrasound treatment.
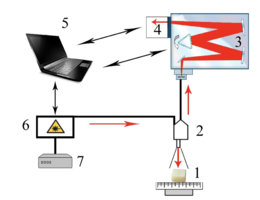
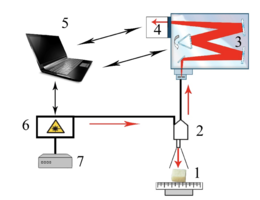
As the main method of research of bioimplants was used the method of Raman spectroscopy, implemented using an experimental stand (figure 1), described in detail in [20].
Raman probe 2 focuses laser radiation on object 1 (spot diameter 0.2 mm at a distance of 7.5 mm from the output window of the tube) and collects the radiation scattered by the object and focuses the radiation filtered in the spectral range 790-1200 nm on the input end of the receiving optical fiber, which is transported to the input slit of the spectrometer Shamrock sr-303i 3 with a built-in cooled chamber 4 with a spectral resolution of 0.15 nm (~1 cm-1). To reduce the noise level, the matrix of the chamber 4 is closed to a temperature of -60oC.
The error of the method does not exceed 4%. Spectra processing was carried out in Wolfram Mathematica 9 software environment and consisted in noise removal by smoothing median filter. Then, on the selected interval 400-2200 cm-1, the
approximating line (fifth degree polynomial) of the autofluorescent component was determined using an iterative algorithm and then this component was subtracted to obtain the selected Raman spectrum.
Figure 1 – Experimental stand : 1 – the object under study; 2 – Raman probe RPB-785; 3 – spectrometer Shamrock sr-303 i; 4 – built-in cooled camera DV420A-OE; 5 – computer; 6 – laser module LuxxMaster LML-785.0 RB-04; 7 – laser module power supply.
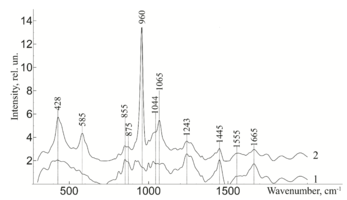
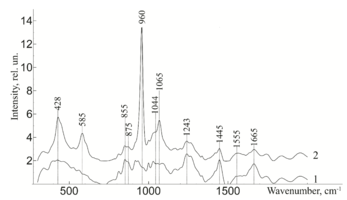
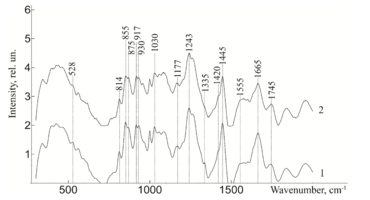
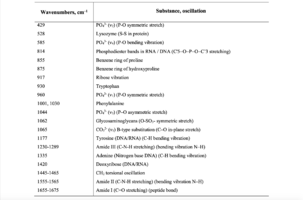
To study the Raman spectra of the greatest interest are the intensities at the wave numbers 960, 1243, 1555, 1665, 855, 1445, 1062, 814, 917 cm-1, corresponding to the components significant for the implant quality: hydroxyapatite, amide III, amide II, amide I, proline, non-collagen proteins and lipids (CH2 torsional oscillation), deformation sulfated glycosaminglican, DNA and RNA. Figure 2 shows the average characteristic Raman spectra for demineralized and mineralized bone tissue samples.
As can be seen from figure 2, samples with different degrees of demineralization have the most significant difference in the bands 960 cm-1 1065 cm-1, 428 and 585 cm-1, as well as on the wave numbers 1243 cm-1, 1555 cm-1, 1665 cm-1, corresponding to the main components determining the quality of implants. The recorded Raman bands correspond to the variations presented in table 1. The Raman spectrum of the mineralized bone tissue sample, in contrast to the demineralized sample, is characterized by pronounced peaks at the wave numbers corresponding to hydroxyapatite: 960 cm-1 (oscillations РО43- (ν1)) and 1065 cm-1 ((СO3)2- (ν1) B-type substitution). The difference between the samples was observed on other Raman lines reflecting the mineral component of hydroxyapatite: 428 cm-1, 585 cm-1 corresponding to the oscillations of phosphate ions.
The obtained data also demonstrate qualitative changes in the composition of bone implants, where the Raman spectra are characterized by an increase in deformation and valence oscillations of the CH2, O-H, C=O, N-H collagen component (amide III 1243 cm-1, amide II 1555 cm-1, amide I 1665 cm-1) [20,21,22], in contrast to non-mineralized samples. That is, there was an increase in the proportion of organic substances, mainly due to a decrease in the mineral component. The difference in the intensity of the Raman bands is explained by the difference in the quantitative content of the main components under study, which is also seen in figure 2. Therefore, the values of Raman peaks can be used as a criterion for controlling the composition of bone implants.
Figure 2 - Raman spectra normalized to the average intensity value for: 1) demineralized bone tissue samples 2) mineralized bone tissue samples
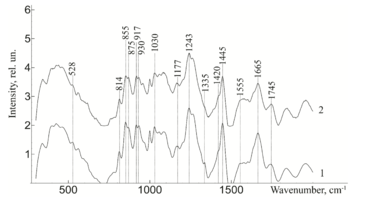
Figure 3 - Raman spectra normalized to the average intensity value for demineralized bone tissue samples: 1) without ultrasonic processing 2) with ultrasonic processing.
The degree of treatment of bone implants is determined by the relative concentration of mineral components, the absence of immunogenic cells, such as bone marrow cells, adipose tissue, and the preservation of glycosaminglican and amide groups in their composition.
Further, from figure 3 it can be seen that the ultrasonic treatment does not have a significant effect on the line of Raman scattering of bone tissue samples 814, 917, 1177, 1420, 855, 875 and 1445 cm-1, corresponding to the content of DNA, RNA, tyrosine, proline, hydroxyproline and non-collagen proteins. The analysis showed that the impact of low-frequency ultrasound in the process of implant manufacturing does not have statistically significant changes in the composition of bone tissue, except for a decrease in the concentration of adenine (nitric base of DNA), therefore, low-frequency ultrasound has a destructive effect on the cellular components of the bone implant. The calculated correlation coefficient between the spectra of samples with and without ultrasound treatment was R=0.992.
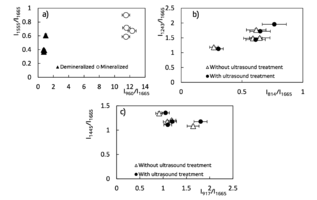
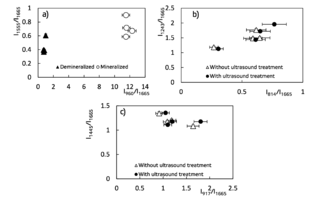
Figure 4 – Two – dimentional diagrams of the input coefficients
The regenerative properties of implants are determined by their structure and composition. Preserved glycosaminoglycans, proline, hydroxyproline and collagen affect its elasticity, hydroxyapatite gives shape and firmness to the bone, and residual RNA and cells can cause immunological rejection of implants. The introduction of optical coefficients is proposed to estimate these parameters. Relatively constant components in the studied bone tissue samples is amide I [23], corresponding to the wave number 1665 cm-1, so it was used as a denominator in the introduced optical coefficients (k):
where Ii – intensity values on wave numbers of analyzed components.
To assess the quantitative content of the main components of bioimplants, a two-dimensional analysis of the introduced optical coefficients was performed, which made it possible to compare the quality of the studied samples. Figure 4 shows two-dimensional diagrams representing the content of the main components of the bone implants, obtained by different processing. A two-dimensional analysis of the optical coefficients was performed to assess the quantitative content of the main components of bioimplants. The coefficients allow to compare the quality of the samples. Figure 4 shows two-dimensional diagrams representing the content of the main components of bone implants obtained by various treatments.
From the analysis of figure 4a, it is seen that the demineralized sample is characterized by a smaller concentration of the main components that also can be seen from figure 2, the values of the coefficient of "mineral/organic matrix" < 1 and the relationship of amide II / amide I.
The values of optical coefficients for bone tissue samples without treatment and with ultrasound treatment are practically the same (figure 4b, 4c) and reflect the similar relative concentration of the main components of the implant: Amide III 1 < k < 2, DNA 0.2 < k < 0.8, RNA 0.9 < k < 1.8 and non-collagenic proteins 1.08 < k < 1.35, that is also shown at the figure 3.
Table 1. Raman bands for the samples of bone tissue
The features of Raman spectra for bone implants, manufactured under the various protocols, were studied. It was found that demineralization is characterized by differences at the wavenumbers 428, 585, 960, 1065, 1243 and 1555 cm-1 corresponding to the significance for quality of the implants: hydroxyapatite and collagen (amide III and amide II). Raman spectroscopy can be used for assessment of bone implants quality during processing. Two-dimensional analysis of the optical coefficients, which allow to estimate bone implants quality, manufactured according to different protocols. It was determined ultrasonic treatment reduces the concentration of cellular components in the bone tissue.
The studies were carried out with financialsupport of Russian Foundation of Basic Research (RFBR), project 18-32-00004.
[1] Mironov, S. P., "Organizational aspects of the problem of osteoporosis in traumatology and orthopedics," Journal of Traumatology and Orthopedics 1, 3-6. (2009).
[2] Rodionova, S. S., "Combined treatment of femoral neck fractures against osteoporosis," Russian Medical Journal 12(24), 1–8 (2004).
[3] Tikhilov, R. М., "The original method of osteosynthesis of the femoral neck with non-free bone autoplasty," Traumatology and orthopedics of Russia 3(61), 91-96 (2011).
[4] Gross, U., "The ultrastructure of the interface between a glass ceramic and bone," J. Biomed. Mater. Res. 15(3),291-305 (1981).
[5] Steinemann, S. G., "Corrosion of surgical implants – in vivo and in vitro tests," Evaluation of Biomaterials 1-34 (1980).
[6] Suchanek, W., "Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants," J. Mater. Res 13(1), 94-117 (1998).
[7] Kirilova, I. А., "Demineralized bone graft as a stimulator of osteogenesis: modern concepts," Spinal Surgery 3, 105–110 (2004).
[8] Popkov, А. V., "Biocompatible implants in traumatology and orthopedics (literature review)," Genius orthopedics3, (2014).
[9] Berchenko, G. N., "Bone grafts in traumatology and orthopedics," Biomaterials 9, 4–5 (2008).
[10] Eppley, B. L., Pietrzak, W. S., Blanton, M. W., "Allograft and alloplastic bone substitutes: a review of science and technology for the craniomaxillofacial surgeon," J Craniofac Surg 16(6), 981–989 (2005).
[11] Esposito, M., "Biological factors contributing to failures of osseointegrated oral implants. (I) Success criteria and epidemiology," European Journal of Oral Sciences 106, 527–551 (1998).
[12] Rashad, A., "Heat production during different ultrasonic and conventional osteotomy preparations for dental implants," Clinical Oral Implants Research 22, 1361–1365 (2011).
[13] Tansik, G., "Glycosaminoglycan mimetic peptide nanofiber gel as an osteoinductive scaffold," Biomater. Sci. 4, 1328 (2016).
[14] Ager III, J. W., "On the Increasing Fragility of Human Teeth with Age: A DeepUV Resonance Raman Study," Journal of Bone and Mineral Research 21(12), 1879–1887 (2006).
[15] Penel, G. L., "Micro-Raman spectral study of the PO4 and CO3 vibrational modes in synthetic and biological apatites," Calcified Tissue International 63, 475-481 (1998).
[16] Draper, E. R. C., "Novel Assessment of Bone Using Time-Resolved Transcutaneous Raman Spectroscopy," Journal of Bone and Mineral Research 20(11), 1968 (2005).
[17] Zakharov, V. P., "Ecological monitoring of megapolis on the basis of differential backscattering control of the wood culture," Laser Physics 19(6), 1366-1372 (2009).
[18] Timchenko, E. V., "Mapping of the Samara city by definition of areas with hydrogen degassing using Raman spectroscopy," Proc. of SPIE 9448(94480K), (2014).
[19] Tarnowski, C.P., "Mineralization of developing mouse calvaria as revealed by Raman microspectroscopy," Journal of Bone and Mineral Research 17(6), 1118 (2002).
[20] Timchenko, P. E., "Application of Raman spectroscopy to assess the condition of bone and cartilaginous biopsy specimens," Journal of Optical Technology 84(6), 423-425 (2017).
[21] Pavlova, Т.V., "Comparative evaluation of the mineral composition and ultramicrostructure of tooth tissues in normal and caries," Sovremennyye naukoyemkiye tekhnologii 12(15), (2009).
[22] Timchenko, P. E., "Optical Analysis of Implants from the Dura Mater," Optical Memory and Neural Networks 27(1), 46–52 (2018).
[23] Jason, R. "Transcutaneous Raman Spectroscopy of Bone," Ph.D., University of Rochester 173 (2013).
-
Chemometric analysis of bioimplants of bone tissues during their manufacture
 Файл статьи P. E. Timchenko, E. V. Timchenko, L. T. Volova, O. O. Frolov, "Chemometric analysis of bioimplants of bone tissues during their manufacture," Proc. SPIE 11363, Tissue Optics and Photonics, 113632A (2 April 2020); doi: 10.1117/12.2565723 Event: SPIE Photonics Europe, 2020, Online Only, FranceПодробнее
Файл статьи P. E. Timchenko, E. V. Timchenko, L. T. Volova, O. O. Frolov, "Chemometric analysis of bioimplants of bone tissues during their manufacture," Proc. SPIE 11363, Tissue Optics and Photonics, 113632A (2 April 2020); doi: 10.1117/12.2565723 Event: SPIE Photonics Europe, 2020, Online Only, FranceПодробнее -
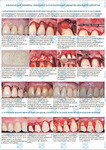
Хирургическое лечение множественных рецессий десны с использованием аллогенной твердой мозговой оболочки «Лиопласт» (Самара) (Лабораторное, Гистологическое, Клиническое, Рентгенологическое исследования)
 Презентация конференции
Хирургическое лечение множественных рецессий десны с использованием аллогенной твердой мозговой оболочки «Лиопласт» (Самара) (Лабораторное, Гистологическое, Клиническое, Рентгенологическое исследования)
Подробнее
Презентация конференции
Хирургическое лечение множественных рецессий десны с использованием аллогенной твердой мозговой оболочки «Лиопласт» (Самара) (Лабораторное, Гистологическое, Клиническое, Рентгенологическое исследования)
Подробнее
-
Клинико-экспериментальное обоснование нового метода хирургического лечения больных с ретенцией нижних третьих моляров
 Цель исследования — клинико-экспериментальное обоснование нового метода хирургического лечения больных с ретенцией нижних третьих моляров
Подробнее
Цель исследования — клинико-экспериментальное обоснование нового метода хирургического лечения больных с ретенцией нижних третьих моляров
Подробнее
-
Оптимизация остеопластичес-кой коррекции атрофирован-ного альвеоляра челюсти. Клинико-экспериментальное исследование
 Цель исследования — разработка но-вого комплекса оперативной коррек-ции атрофированных альвеоляров челюстей для дентальной имплантации и протезирования
Подробнее
Цель исследования — разработка но-вого комплекса оперативной коррек-ции атрофированных альвеоляров челюстей для дентальной имплантации и протезирования
Подробнее