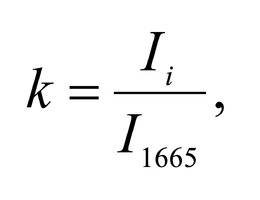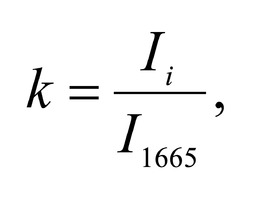+7 (929) 727 53 60 Травматология / Ортопедия
Raman spectroscopy method for the evaluation of bone bioimplants made using the "Lyoplast" technology from cadaveric and in vivo resected bone tissue
P. Е Timchenko1, Е. V Timchenko1, L. Т Volova2, D. А Dolgushkin, V. V Boltovskaya, О. О Frolov1
1 — Samara National Research University, 34, Moskovskoye shosse, Samara, 443086, Russia
2 — Samara State Medical University, 89, Chapayevskaya St., Samara, 443099, Russia
E-mail: laser-optics.timchenko@mail.ru
The results of application of the Raman spectroscopy method for estimating alternative sources for the production of bone spongy implants using the "Lioplast" technology, namely, the femoral head heads resected in the operation of hip replacement surgery, are presented. It is shown that Raman spectroscopy can be used to assess the component composition of the bone implants surface during their processing. Comparing different sources of sponge bone production before and after demineralization, no significant differences were found, but there are differences in the ratio of the Raman peaks intensities at wave numbers 1555 cm-1 and 1665 cm-1 corresponding to amide II and amide I, and also in the intensity of Raman peaks at the wave numbers 429 cm-1 (PO4 3- (ν2) (PO symmetric vibrational)), 1068 cm-1 (СO3 2- (ν1) B-type substitution (C-O planar valence)), 850 cm-1 (benzene ring proline), 1000 cm-1 , (aromatic phenylalanine ring).
Recently, reconstructive and regenerative medicine is actively developing. In traumatology, orthopedics, dentistry, oncology and purulent surgery the search for approaches to the treatment of the supporting and integumentary tissues pathology of the human body assumes not only the means of its elimination, but also the preservation of the original form, structure and functions destroyed by traumas and bone diseases [1, 2]. Providing full regeneration of bone tissue in the defective bone sites area, in spite of the accumulated knowledge in this matter – is one of the most acute problems of modern medicine. It can be solved by creating optimal conditions for regenerative processes in the zones of its resorption. One of the ways is the use of osteoplastic materials [3, 4]. Among them, allogeneic implants from human tissues are the optimal materials for the reconstruction of musculoskeletal system injuries. When applied, unlike auto– and xenoplasty and the use of synthetic drugs, the homeostasis and metabolism of connective tissues and the functions of the life–support systems of the recipient are not impaired [5]. Allogenic materials after their special treatment almost completely lose their antigenicity and when placed in the body do not have a negative impact on it. They play the role of a matrix, conductor, gradually dissolve completely, and in their place a new bone tissue is formed [6]. With the increase in the number of operations performed in the region of hip arthroplasty, it became possible to use resectable intraoperatively femoral heads to produce new bone spongy bioimplants using the "Lioplast" technology.To compare the quality of bioimplants obtained from cadavers and resected intraoperatively spongy bones treated with the Lioplast technology, it is advisable to use physical methods of investigation. Raman spectroscopy has certain advantages, allows real-time non-invasive quantitative and qualitative analysis of the composition of biological objects, and provides information on the molecular structure with high spatial resolution. For example, in [7, 8, 9], the Raman spectroscopy method was used to evaluate the content of key components of human bone tissue – hydroxyapatite and amides of the I–III type. Comparing the amplitudes of the Raman peaks at wavenumbers in the range from 400 to 1800 cm-1 , the authors revealed significant signal changes for samples with different degrees of demineralization. The aim of this study: a comparative assessment of the component composition of mineralized and demineralized bone bioimplants surfaces manufactured using the "Lioplast" technology from cadaveric and in vivo resected spongy bone.
MATERIALS AND METHODS OF RESEARCH
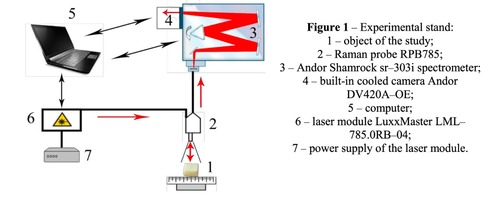
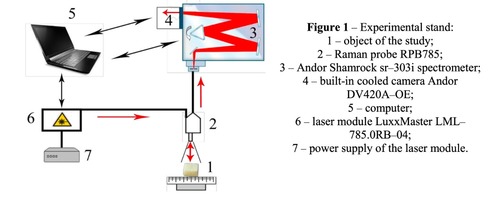
Materials of the study were samples of a spongy bone bioimplants in the form of a cube 5 * 5 * 5 mm in size, manufactured using the "Lioplast" ® technology (TU–9398–001–01963143–2004). The samples were divided into four groups. The first group consisted of demineralized samples obtained from cadaveric tissue, the second group – mineralized samples from the same source. The third group formed demineralized samples made from the femoral head heads obtained intraoperatively in hip replacement, the fourth group – mineralized samples from the same source. In the process of bone bioimplants production, at the first stage, they performed their low-frequency ultrasound treatment (24–40 kHz for 2-3 minutes), as a result of which the samples were degreased, all the elements of the bone marrow were removed from the interballal spaces of the bone. To obtain demineralized samples, bone tissue was placed in a hydrochloric acid solution of 2.4 normality. At the final stage of the treatment, lyophilization of the bones was carried out by means of the ALPHA 2–4 LSC, which consisted in freeze drying of the samples. As a basic method for studying bioimplants, the Raman spectroscopy method was used, realized using an experimental stand (Figure 1), described in detail in the work [10]. The spectra were taken for each sample on each side of the cube at three different points.The Raman probe 2 focused laser radiation at object 1 (spot diameter 0.2 mm at a distance of 7.5 mm from the exit window of the tube), collected the radiation scattered by the object and focused the radiation filtered out in the spectral range 790–1200 nm to the input end of the receiving optical fiber, through which it was transported to input slit of the Shamrock sr-303i 3 spectrometer with integrated cooling chamber 4. To reduce the noise level, the camera array 4 was frozen to a temperature of -60ºC, providing a spectral resolution of 0.15 nm (~ 1 cm-1 ). The error of the method did not exceed 4%. Spectrum processing was carried out in the software environment Wolfram Mathematica 10 and consisted in removing the noise by a smoothing median filter of 7 points. Then, on the selected interval 400-2200 cm-1 , using the iterative algorithm [11], an approximating line (a polynomial of the fifth degree) of the auto-fluorescent component was determined, and then this component was subtracted, obtaining a separate Raman spectrum.
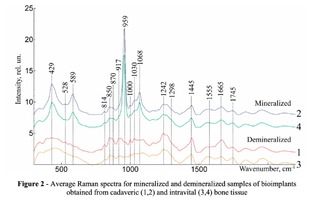
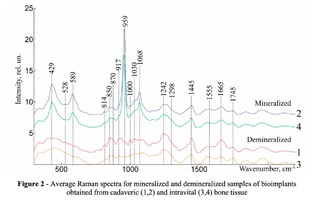
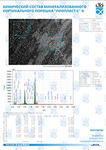
To study the Raman spectra of bones the intensities at the wavenumbers of 959 cm-1 (vibrations of the phosphate ion PO4 3- (ν1) (P–O symmetric valence)), 1068 cm-1 (carbonate ion CO3 2- (ν1) B-type substitution (С–О in-plane stretch)), 1555 cm-1 (amide II (C–N–H valence) (deformation vibration N– H)), 1665 cm-1 (amide I (C=O valence) (peptide bond)), 850 cm-1 (a benzene ring of proline), 870 cm-1 (a benzene ring of hydroxyproline), 1000 cm-1 (aromatic ring breathing of phenylalanine (protein assignment)), 1030 cm-1 (phenylalanine (CH2CH3 bending modes) (collagen assignment)), 917 cm-1 (ribose (RNA)) and 1298 cm-1 (fatty acids (CH2, CH3 twisting and wagging) (lipids)) were chosen [7, 9, 12]. Figure 2 shows the averaged Raman spectra for mineralized and demineralized samples obtained from different sources.The interpretation of Raman spectra for samples of bone bioimplants is presented in Table 1.As can be seen from Figure 2, significant differences in the intensities of the wavenumber peaks when comparing bioimplants, for the production of which bone tissue from different sources was used, was not found. For mineralized bioimplants of both groups, was found an increase in the intensities at the wavenumbers of 959 cm-1 , 1068 cm-1 , 429 and 589 cm-1 , corresponding to PO4 3- (ν1) (P–O symmetric valence), CO3 2- (ν1) substitution B-type, PO4 3- (ν2) (PO symmetric vibrational), PO4 3- (ν4) (PO deformation.) After demineralization a decrease in the intensity was observed at these wave numbers both in samples obtained from cadaveric and in vivo. For mineralized specimens the correlation coefficient between both groups was in the range of Rm = {0.924; 0.987}. The calculated correlation coefficient for samples of demineralized bioimplants of both groups was Rd = {0.834, 0.963}. It should be noted that in all samples of bone bioimplants manufactured using the Lioplast technology, no high intensity was observed at wave numbers – 1298 cm-1 , 1745 cm-1 , corresponding to fatty acids (CH2, CH3 twisting and wagging (lipids)) and C=O valence) phospholipids. The use of lowfrequency ultrasound at the stages of spongy bone treatment made it possible to maximize the removal of lipids and bone marrow elements, which was confirmed in the spectral characteristics of the bioimplant samples surfaces under study. Thus, according to the results of the Raman spectra analysis, the source of bone tissue production (cadaveric or intravital spongy bone) did not affect the component composition of the implants surface created using the Lioplast technology. However, for a more complete quantitative assessment of the component composition of the bone bioimplants surface, we introduced optical coefficients. A relatively constant component in the samples of bone tissue studied was amide I [15], corresponding to a wave number of 1665 cm-1 , so it was used as a denominator in the introduced optical coefficients (k):
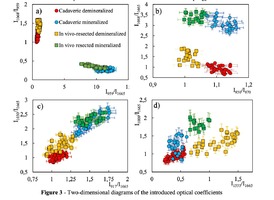
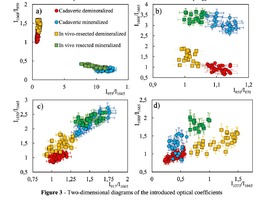
To analyze the organomineral composition of bone tissue and biomaterials based on hydroxyapatite, the following ratios can be used: The ratio I959/I1665 determines the degree of leaching of mineral components in the process of demineralization [16]. The optical coefficient I1068/I1665 (Carbonate / Organic Matrix ratio) is used to estimate the degree of carbonate mineralization of the biomaterial [16].Both coefficients show the dependence of the mineral content in relation to the organic component. I1555/I1665 coefficient (ratio Amide II / Amide I) characterizes the change in Amide I with respect to Amide II [17]. I1068/I1665 and I1068/I959 coefficients (ratio of Carbonate / Amide I and Carbonate / Phosphate) indicate the degree of carbonate inclusion in the crystal lattice of hydroxyapatite [17]. The ratio I850/I870 (proline / hydroxyproline) is an indicator of damage to the structure of the collagen helix with a decrease in the relative concentration of proline [18]. A two-dimensional analysis of the introduced optical coefficients was carried out. Figure 3 shows two-dimensional diagrams representing the quantitative content of the main components of mineralized and demineralized bone implants made from cadaveric and in vivo resected spongy bones.It can be seen from the analysis of figure 3a that for demineralized samples a smaller value of the
coefficient I959/I1665 – "mineral / organic matrix" (0.8 < I959/I1665 <1.3) was characteristic, compared to
mineralized samples (7
the values of the I959/I1665 coefficient for demineralized tissue samples were close, despite the difference
in the sources of spongy bone sampling for the manufacture of implants.
The I1068/I1665 ratio (Carbonate / Organic Matrix ratio) was used to assess the degree of mineralization
of biomaterials. It can be seen from figure 3b that for the demineralized samples the coefficient I1068/I1665
varies within the limits of 0.5 < I1068/I1665 < 2) and for mineralized samples in the range 2.5 < I1068/I1665
< 4.Also it can be seen from Figures 3a, 3b that higher values of I1068/I959 and I1068/I1665 coefficients are characteristic for samples obtained from intravital resected bone tissue than for cadaveric bone, and, consequently, a higher content of B–type carbonate carbonate CO3 2- (ν1), which is more resistant to the effect of acid during demineralization [17], which manifests itself in an increase in the difference between materials in the process of demineralization for the coefficients I1068/I959 and I1068/I1665. The ratio I850/I870 shows the difference in the ratio of the peaks of proline and hydroxyproline benzene rings due to deformations in the structure of collagen [18], and allows differentiating cadavers (1.07 < I850/I870 <1.16) and samples from intravital resected bone tissue (0.98 < I850/I870 < 1.05). For demineralized and mineralized samples, the values of the ratio I850/I870 were in the same intervals. The process of bone implants demineralization using the "Lioplast" technology made it possible to lower the level of the content of such a bone component as ribose (RNA), which reflected the change in the optical coefficient I917/I1665 from 1.3 < I917/I1665 < 1.75 to 0.9 < I917/I1665 < 1.25 after demineralization. The values of the coefficient I917/I1665 did not depend on the source of bone tissue production. Reducing the amount of RNA during processing of biomaterials using the "Lioplast" technology reduced their immunogenicity for the recipient organism. The values of the optical coefficient I1030/I1665 vary from the interval 0.8 < I1030/I1665 <1.6 for demineralized and to the interval 1.7 < I1030/I1665 < 2.6 for mineralized samples (figure 3c), which reflects a decrease in the relative concentration of phenylalanine with respect to secondary protein structures (amide I) in the process of demineralization, and for the samples obtained from intravital resected bone tissue this process proceeds more slowly. Analysis of diagram 3d shows that the value of the optical coefficient I1555/I1665 does not significantly depend on the degree of demineralization of the samples, but there is a separation of the regions for samples obtained from the femoral head in the intraoperative (0.66 < I1555/I1665 < 1.5) and cadaveric 0.2 < I1555/I1665 < 0.6).
A comparative spectral evaluation of the surfaces component composition of mineralized and demineralized bone bioimplants manufactured using the "Lioplast" technology from cadaveric tissue and femoral bone heads resected during the operation of hip arthroplasty was performed. Comparing different sources of sponge bone production before and after demineralization, no significant differences were found, but there are differences in the ratio of the Raman peaks intensities at wave numbers 1555 cm-1 and 1665 cm-1 corresponding to amide II and amide I, and also in the intensity of Raman peaks at the wave numbers 429 cm-1 (PO4 3- (ν2) (P–O symmetrical vibrational)), 1068 cm-1 (CO3 2- (ν1) B-type substitution (C–O planar valence), 850 cm-1 (benzene ring of proline), 1000 cm-1 (aromatic ring of phenylalanine). Optical coefficients were introduced and a two-dimensional analysis was carried out, which showed that the values of I1068/I959 and I1068/I1665 are higher for intravital resected bone tissue than for cadaveric bone, and therefore a higher content of B-type carbonate carbonate CO3 2- (ν1), which is more resistant to the effects of acid during demineralization. The introduced optical coefficients made it possible to confirm that the content of the main components of bioimplants necessary for the realization of their osteoinductive and osteoconductive properties turned out to be similar, both in mineralized and demineralized samples obtained from the femoral head intraoperatively, and from samples made from cadaveric material.
The reported study was funded by RFBR according to the research project № 18-32-00004.
[1] Popkov A V 2014 Biocompatible implants in traumatology and orthopedics (review of the literature) (Geniy ortopedii) №3
[2] Berchenko G N 2008 Bone grafts in traumatology and orthopedics (Biomaterials) №9 p. 4–5
[3] Muslimov S А 2000 Morphological Aspects of Regenerative Surgery (Ufa: Bashkortostan) p. 168
[4] Lekishvili М V 2005 Technologies for manufacturing bone plastic material for use in reconstructive surgery (dissertation of Doctor of Medical Sciences: 14.00.41, 14.00.22 Moscow) p. 47
[5] Saveliev V I, Kornilov N V, Ivankin D Е, Linnik S А 2001 Allotransplantation of formalinized bone tissue in traumatology and orthopedics (Saint Petersburg, MORSARAB) p. 208
[6] Ladonin S V, Belozertseva Е А 2007 Application of allogeneic demineralized bone implants in the treatment of chronic osteomyelitis in an experiment (Topical issues of tissue and cell transplantology: Moscow, CITO) p. 27
[7] Ager III J W, Nalla R K, Balooch G, Kim G, Pugach M, Habelitz S, Marshall G W, Kinney J H, O’Ritchie R 2006 On the Increasing Fragility of Human Teeth With Age:A Deep-UV Resonance Raman Study (Journal of Bone and Mineral Research vol 21) pp. 1879-1887
[8] Draper E R C, Morris M D, Camacho N P, Matousek P, Towrie M, Parker A W, Goodship A E 2005 Novel Assessment of Bone Using Time-Resolved Transcutaneous Raman Spectroscopy (Journal of Bone and Mineral Research) 20 (11), p. 1968
[9] Tarnowski C P, Ignelzi Jr M A, Morris M D 2002 Mineralization of Developing Mouse Calvaria as Revealed by Raman Microspectroscopy // (Journal of Bone and Mineral Research) 17, № 6. pp 1118– 1126
[10] Timchenko Е V, Timchenko P Е, Volova L Т, Ponomareva Yu V, Taskina L А 2014 Investigation of the organomineral structure of bone implants by Raman scattering (Quantum Electronics) 44 (7), pp. 696 - 700
[11] Zhao J, Lui H, Mclean D I, Zeng H 2007 Automated autofluorescence background subtraction algorithm for biomedical Raman spectroscopy (Society for applied spectroscopy) 61(11), pp. 1225– 1232
[12] Moaghi Z, Rehman S, Rehman I 2007 Raman Spectroscopy of Biological Tissues (Applied Spectroscopy Reviews) 42, pp. 493–541
[13] Olsztyńska-Janus S, Gasior-Glogowska M, Szymborska-Malek K, Komorowska M, Witkiewicz W, Pezowicz C, Szotek S, Kobielarz M 2012 Spectroscopic techniques in the study of human tissues and their components. Part II (Raman spectroscopy Acta of Bioengineering and Biomechanics) Vol. 14, № 4
[14] Polomska M, Kubisz L, Kalawski R, Oszkinis G, Filipiak R, Mazurek A 2010 Fourier Transform Near Infrared Raman Spectroscopy in Studies on Connective Tissue (Acoustic and Biomedical Engineering) Vol.118 №1
[15] Maher J R, 2013 Transcutaneous Raman Spectroscopy of Bone (University of rochester) 173 p.
[16] Timchenko P Е, Timchenko Е V, Pisareva Е V, Vlasov М Yu, Volova L Т, Frolov О О 2017 Experimental studies of hydroxyapatite (Journal of Optical Technology)
[17] Lim N S, Hamed Z, Yeow C H, Chan C, Huang Z 2011 Early detection of biomolecular changes in disrupted porcine cartilage using polarized Raman spectroscopy (J. Biomed. Opt.) 16 (1): 017003
-
Raman spectroscopy for evaluation of dura mater based graft
 Файл статьи P. Е Timchenko, Е. V Timchenko, L. Т Volova, О. О Frolov, А. U Kulabuhova, N. K KiykoПодробнее
Файл статьи P. Е Timchenko, Е. V Timchenko, L. Т Volova, О. О Frolov, А. U Kulabuhova, N. K KiykoПодробнее -
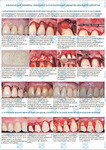
Хирургическое лечение множественных рецессий десны с комбинированным применением аутотрансплантата и аллогенной лиофилизированной dura mater: клинический случай
 Файл статьи
М. А. Носова1, Л. Т. Волова1, А. Н. Шаров2, Д.А. Трунин1, М. А. Постников1
Подробнее
Файл статьи
М. А. Носова1, Л. Т. Волова1, А. Н. Шаров2, Д.А. Трунин1, М. А. Постников1
Подробнее
-
Эффективность применения аллогенной dura mater для превентивного хирургического лечения образования одиночных и множественных рецессий десны перед ортодонтическим лечением несъемной ортодонтической техникой: клиническое исследование
 Файл статьи
ПодробнееМ.А. Носова1, Д.Д. Березина3, Л.Т. Волова1, А.Н. Шаров2, Д.А. Трунин1, М.А. Постников1
Файл статьи
ПодробнееМ.А. Носова1, Д.Д. Березина3, Л.Т. Волова1, А.Н. Шаров2, Д.А. Трунин1, М.А. Постников1 -
Доклад на IV Международной научно-практической конференции «Современная гнатология»
 Файл анонса
М.А. Носова, Д.Д. Березина, Л.Т. Волова, А.Н. Шаров, Д.А. Трунин, М.А. Постников
Подробнее
Файл анонса
М.А. Носова, Д.Д. Березина, Л.Т. Волова, А.Н. Шаров, Д.А. Трунин, М.А. Постников
Подробнее