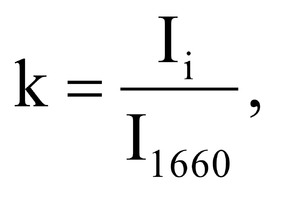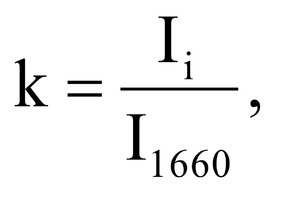+7 (929) 727 53 60 Травматология / Ортопедия
Research of component composition of mineralized bone implants by Raman spectroscopy
P.E. Timchenko1, E.V. Timchenko*1, L.T. Volova2, O.O. Frolov1, V.D. Meseyarakov1, Pugachov E.I.2
1 Samara National Research University, 34, Moskovskoye shosse, Samara, 443086, Russia
2 Samara State Medical University, 89 Chapayevskaya St., Samara, 443099, Russia
The results of Raman spectroscopy to evaluate alternative sources for donor bone implants using the "Lioplast" technology are presented. It is shown that Raman spectroscopy can be used to assess the component composition of the surface of bone implants during their treatment. It has been found that major differences were at Raman bands 1448, 1735 (lipids & fatty acid), 850 and 875 cm-1 (proline & hydroxyproline), 1001 and 1026 cm-1 (phenylalanine) and 1272, 1560 cm-1 (amide II, amide III). The criteria which allow to evaluate the component composition in the production of bioimplants are proposed, and a two-dimensional analysis was carried out. The analysis showed that the main components of the extracellular matrix necessary for the implementation of osteoinductive and osteoconductive properties of biomaterials are saved during processing. Lipids were removed that improved the quality of the material, providing the possibility of a good clinical effect.
Bioimplants from human tissues are widely used for reconstruction and restoration of the structure of the supporting and conjunctive tissue of the recipient. In surgical and traumatological practice, damages of the integrity of bone tissue related to diseases of the musculoskeletal system and emergence of micro-fractures on the background of weakening in bone remodeling with a further increase in the fragility and porosity of the bone is characteristic of a significant percentage of patients. One of the ways to replace bone defects is the use of autogenous bone grafts [1, 2]. Among them, allogeneic implants, which made of human tissues, are optimal materials for the reconstruction of injuries of the musculoskeletal system [3]. Specialists refuse from transplant in the early stages of the study and use of autogenous tissues in the process of transplantation due to the problem of infectious complications after transplantation. However, with the advent of innovative standards for the manufacture of allogeneic materials, after their special treatment, the removal of cellular components (DNA, RNA) is the main factor of antigenicity, and when placed in the body hasn’t a negative impact on it [4]. The quality of the produced bioimplants is evaluated by the content of the necessary biological substances involved in the regenerative process, such as hydroxyapatite, collagen, glycosaminoglycans and proteoglycans [5, 6]. Constant monitoring and quality control are necessary in the process of bioimplants manufacturing. Currently biochemical and morphological methods are used to assess the quality of bioimplants and often require lots of time and resources, which affects the quality of medical care to patients. Therefore, the use of optical methods is promising, because they can be used as screening studies, quickly performed, low-cost and non-destructive for the samples [7, 8, 9]. The aim of this work is determining the most important criteria for the evaluation of mineralized bone implants at the stages of their manufacture using the method of Raman spectroscopy.
MATERIALS AND METHODS RESEARCH
Raman spectroscopy method was chosen as the major method of analysis of the bioimplants. The laboratory facility consisting of a Raman probe RPB-785, combined with a laser module LuxxMaster LML-785.0RB-04 and a high-resolution digital spectrometer Shamrock sr-303i, providing spectral resolution 0,15 nm, with a built-in cooling chamber DV420A-OE [7]. Objects of the research were 48 spongy bone bio-implants in the form of a cube 5*5*5 mm, made using the "Lioplast"® technology ((ТУ-9398-001-01963143-2004). For comparison, the samples were grouped according to the method of preparation: allogeneic (cadaveric) and intraoperatively resected. In each group the samples were divided into 4 subgroups according to the degree of demineralization and ultrasonic treatment (8 groups of samples).
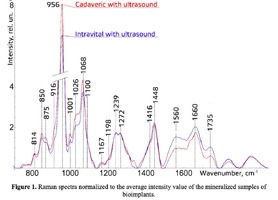
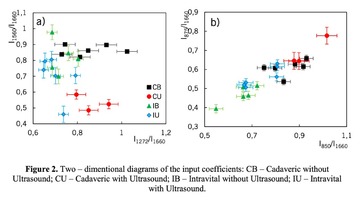
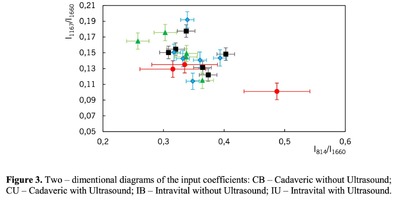
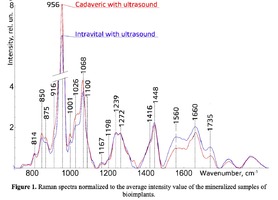
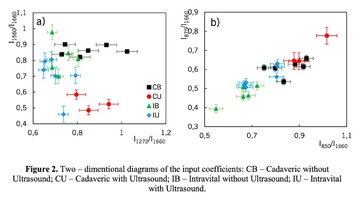
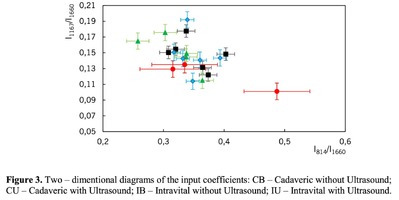
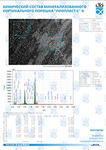
Figure 1 shows the Raman spectra of the mineralized samples. The main differences were found at the Raman bands 875, 956, 1001, 1026, 1068, 1560 cm-1 and 1735 cm-1. Decoding of the Raman spectra is given in table. The change of the intensity at lines 956 and 1068 cm-1 shows the difference in the relative concentration of hydroxyapatite in cadaver and intraoperative samples with ultrasonic treatment. And in the first group the intensity of these lines is higher. The introduced coefficients allow monitoring the relative component composition of surface of the bioimplants. A relative constant component in the studied samples during various processes of physical and chemical effects on bone tissue was amide [5] (figure 1), corresponding to the Raman frequency shift of 1660 cm-1. The intensity of this line was used as a denominator (I1660) in the introduced coefficients (k).where Ii – intensity values of the spectral lines of the analyzed components. A detailed analysis is given in figures 2 - 3, which presents two-dimensional diagrams of the introduced coefficients, shows the differences and similarities between the samples of the four groups.The values of the k1560 coefficient (figure 2a) reflect the relative concentration of amide II. Amide II content is typical for groups of samples without ultrasonic treatment and cadaveric bone tissue. The values of the k1560 coefficient are higher on average than for intraoperative samples and after the ultrasonic treatment. The k1272 coefficient, reflecting the relative concentration of amide III, is higher for cadaver materials and hasn’t a significant influence by ultrasonic treatment. Relative concentration of proline and hydroxyproline is higher for the samples with ultrasonic treatment and for cadaveric biomaterials, the values of the corresponding coefficients k850 and k875 are higher than for the intraoperative samples (figure 2b). Figure 3 shows that the intensity at the bands 814 cm-1 and 1167 cm-1, corresponding to DNA and glycosaminoglycans, remain almost unchanged in the process of ultrasonic treatment. It speaks about impossibility of a complete withdrawal of the remnants of DNA from the samples using the primary sterilization by ultrasound. The observation notices the constancy of the relative concentration of GAGs, CSPGs in relation to collagen structures (amide I) in the manufacturing process, which points on a safety of the components that play an important role in the process of engraftment implant, and obtaining quality extracellular matrix. Thus, it is shown that the use of low-frequency ultrasound at the stages of processing of bioimplants allows the achievement lipid removal, which was confirmed by the spectral characteristics of surfaces of the studied bioimplants.
A comparative spectral assessment of the component composition of the surfaces of implant samples on the basis of mineralized bone tissue, various sources of production, made by "Lioplast" technology, with and without ultrasonic treatment, is carried out. It was found that the main differences are shown at the Raman lines 1448, 1735 (lipids & fatty acid), 850 and 875 cm-1 (proline & hydroxyproline), 1001 and 1026 cm-1 (phenylalanine) and 1272, 1560 cm-1 (amide II, amide III). Thus, the optical coefficients were introduced and the two-dimensional analysis was carried out. The charts showed that the main components of extracellular matrix for the implementation of osteoinductive and osteoconductive properties of biomaterials are preserved during processing, and lipids are removed. It improves the quality of the material that provides possibility of a good clinical effect.
The studies were carried out with financial support of Russian Foundation of Basic Research (RFBR), project 18-32-00004.
[1] Muslimov S А 2000 Morphological Aspects of Regenerative Surgery (Ufa: Bashkortostan) p 168
[2] Lekishvili М V 2005 Technologies for manufacturing bone plastic material for use in reconstructive surgery (Moscow: Dis. Cand. Honey Sciences 14.00.41) p 47
[3] Saveliev V I, Kornilov N V, Ivankin D Е and Linnik S А 2001 Allotransplantation of formalinized bone tissue in traumatology and orthopedics (Saint Petersburg, MORSARAB) p 208
[4] Ladonin S V and Belozertseva Е А 2007 Application of allogeneic demineralized bone implants in the treatment of chronic osteomyelitis in an experiment Topical issues of tissue and cell transplantology: Moscow CITO (Moscow) p 27
[5] Chen H, Xu P W and Broderick N 2016 In vivo spinal nerve sensing in MISS using Raman spectroscopy In Proc. of SPIE (Society of Photo-optical Instrumentation Engineers) 9802 98021L
[6] Chen J L, Duan L and Zhu W 2014 Extracellular matrix production in vitro in cartilage tissue engineering Journal TranslMed 12 88
[7] Timchenko E V, Tregub N V, Taskina L A, Selezneva E A and Timchenko P E 2014 Optical methods for control of hydrogen influence on plants In Proc. of SPIE (Society of Photo-optical Instrumentation Engineers) 9221 922108
[8] Zaharov V P, Timchenko E V, Timchenko P E, Zolotuhina A D and Alembekov S V 2011 Alteration of hydrosphere optical properties by synthetic active compounds Journal Computer Optics 35 238-42
[9] Timchenko P E, Zakharov V P, Volova L T, Boltovskay V V and Timchenko E V 2011 Diagnostics of bone implantat and control of their process osteointegration with of a method confocal microscopy Journal Computer Optics 35 183-7
[10] Moaghi Z, Rehman S and Rehman I 2007 Raman Spectroscopy of Biological Tissues Journal Applied Spectroscopy Reviews 42 493-541
[11] Ruiz-Chica A J, Medina M A, Sanchez-Jimenez F and Ramirez F J 2004 Characterization by Raman spectroscopy of conformational changes on guaninecytosine and adenine-thymine oligonucleotides induced by aminooxy analogues of spermidine Journal of Raman Spectroscopy 35 93–100
[12] Muntean C M, Halmagyi A, Puiac M D and Pavel I 2009 FT-Raman signatures of genomic DNA from plant tissues Spectroscopy 23 59–70
[13] Olsztyńska-Janus S, Gasior-Glogowska M, Szymborska-Malek K, Komorowska M, Witkiewicz W, Pezowicz C, Szotek S and Kobielarz M 2012 Spectroscopic techniques in the study of human tissues and their components. Part II Journal Raman spectroscopy Acta of Bioengineering and Biomechanics 14 121–33
[14] Cheng W-T, Liu M-T, Liu H-N and Lin S-Y 2005 Micro-Raman spectroscopy used to identify and grade human skin pilomatrixoma Journal Microscopy Research and Technique 68 75-9
[15] Timchenko E V, Timchenko P E, Volova L T, Dolgushkin D A, Shalkovsky P Y and Pershutkina S V 2016 Detailed spectral analysis of decellularized skin implants J. Phys.: Conf. Ser. 737 012050 1-4
[16] Timchenko P E, Timchenko E V, Pisareva E V, Vlasov M Yu, Volova L T, Frolov O O and Kalimullina A R 2018 Experimental studies of hydroxyapatite by Raman spectroscopy Journal of optical technology 85 130-5
-
Raman spectroscopy for evaluation of dura mater based grafts
 Файл статьи P. Е Timchenko, Е. V Timchenko, L. Т Volova, О. О Frolov, А. U Kulabuhova, N. K KiykoПодробнее
Файл статьи P. Е Timchenko, Е. V Timchenko, L. Т Volova, О. О Frolov, А. U Kulabuhova, N. K KiykoПодробнее -
Analysis of the mineral component for cortical bone tissue by Raman spectroscopy after ovariectomy and its treatment with allogeneic hydroxyapatite
 Файл статьи E. Timchenko1, P. Timchenko1, E. Pisareva1, M. Vlasov2, L. Volova2, I. Fedorova1, A. Tumchenkova1, M. Gorchenkova1 and A. Subatovich1Подробнее
Файл статьи E. Timchenko1, P. Timchenko1, E. Pisareva1, M. Vlasov2, L. Volova2, I. Fedorova1, A. Tumchenkova1, M. Gorchenkova1 and A. Subatovich1Подробнее -
Research of component composition of bioimplants for treatment of gum recession using a Raman spectroscopy method
 Файл статьи P. Е. Timchenko1, Е. V. Timchenko1, L. Т. Volova2, О. О. Frolov1 and E. F. Yagofarova1Подробнее
Файл статьи P. Е. Timchenko1, Е. V. Timchenko1, L. Т. Volova2, О. О. Frolov1 and E. F. Yagofarova1Подробнее -
Raman spectroscopy method for the evaluation of bone bioimplants made using the "Lyoplast" technology from cadaveric and in vivo resected bone tissue
 Файл статьи P. Е Timchenko1, Е. V Timchenko1, L. Т Volova2, D. А Dolgushkin , V. V Boltovskaya , О. О Frolov1Подробнее
Файл статьи P. Е Timchenko1, Е. V Timchenko1, L. Т Volova2, D. А Dolgushkin , V. V Boltovskaya , О. О Frolov1Подробнее